The study of matter is generally characterised as chemistry. Terms like substance, compound, element, combination, and others are associated with the idea of matter. Matter can also be characterised by its qualities, such as smell and colour. Physical and chemical qualities are distinguished. Changes in substances can occur, some of which are characterised as physical changes and others as chemical changes.
The evolving concepts and systems employed to describe and explain the material world are covered by matter theory. A theory of the elements underpins a substantial portion of matter theory. The three states of matter usually found on Earth are solids, liquids, and gases. This article will help students learn more about the classification, physical properties, and chemical properties in this article.
Classifying Matter
Pure substances
The makeup of a pure material is always the same. A pure substance’s composition and qualities are the same in all examples. Elements and compounds are the two categories of pure substances. Elements are pure compounds that cannot be broken down into simpler ones by chemical processes. Iron, silver, gold, aluminium, sulphur, oxygen, and copper are just a few of the more than 100 known elements, of which roughly 90 are found naturally on Earth, and another two dozen or so have been synthesised in labs. Pure substances composed of two or more elements are known as compounds. Chemical changes can break down materials to produce elements, other compounds, or both.
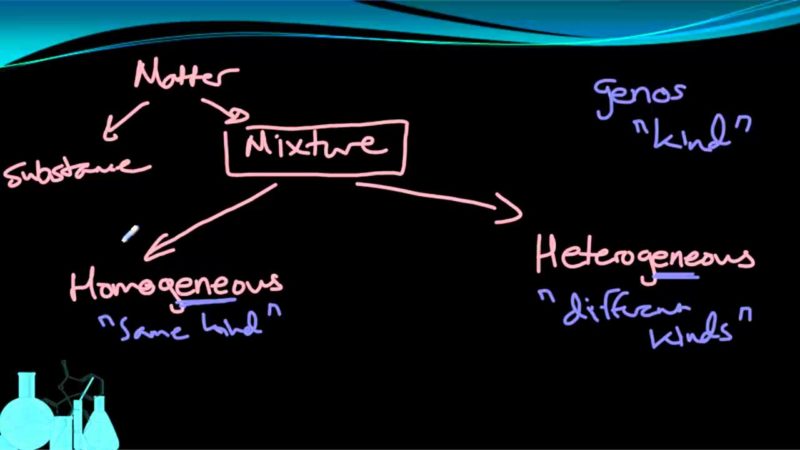
- A substance is defined as a homogeneous matter and has all of the same components. Either elements or compounds are substances.
- Elements are pure compounds that cannot be decomposed chemically into simpler things. Oxygen, gold, sulphur, and iron are all well-known elements.
- Pure substances made up of two or more elements are known as compounds. Compounds include basic substances like water, salt, and sugar.
- On the other hand, wood or a chunk of marble is not homogenous and is not pure substances or compounds. Mixtures are what they’re called.
Mixtures
A mixture is a substance that contains two or more constituents. Individual compounds in a combination retain their chemical identities. A mixture of sand and water, for example, is an evident combination of two or more components. Heterogeneous mixes are such mixtures. Some combinations include closely mixed components that act as if they are one substance (even though they are not). Homogeneous mixes are those that have a uniform composition throughout (or solutions). A solution is anything like sugar dissolved in water. A solid solution, such as steel, is an example of a metal alloy. Air is a gaseous solution made up mostly of nitrogen and oxygen.
Physical Properties
- The matter is made up of microscopic particles known as atoms, and it may be represented or interpreted as anything that occupies space. The matter must show both the mass and volume characteristics.
- Properties are the characteristics that allow us to differentiate one material from another. A physical property is a characteristic of matter that is unrelated to its chemical makeup.
- Physical qualities include density, colour, hardness, melting and boiling temperatures, and electrical conductivity.
Solid, liquid, and gas are the three basic phases of matter. The most matter may exist in any of these states, depending on its physical characteristics. Scientists work with a wide variety of materials in particular. Properties of matter refer to any feature that can be measured, such as an object’s density, colour, mass, volume, length, malleability, melting point, hardness, odour, temperature, and so on.
Chemical Properties
- Reactivity is the property of matter that allows it to mix chemically with other substances. Certain materials have high reactivity, whereas others have low reactivity. Even in the presence of water, potassium, for example, is exceedingly reactive. When a pea-sized amount of potassium is mixed with a little amount of water, it explodes.
- Flammability is the property of a substance that allows it to burn. When matter burns, it interacts with oxygen to produce a variety of compounds. A flammable substance is anything that burns, such as wood.
- The degree to which a chemical element or a mixture of chemicals may harm an organism is referred to as toxicity.
- Acidity is a chemical attribute that describes a substance’s capacity to react with an acid. When some metals react with various acids, they generate compounds. Acids and bases combine to form water, which neutralises the acid.
Summary
The study of matter’s composition and the transition is referred to as chemistry. The substance is a phrase that is commonly used interchangeably with the matter, although it has a more limited definition in chemistry. Chemistry also studies the behaviour of matter. Chemistry is concerned with the composition, structure, and characteristics of matter, as well as the phenomena that occur when various types of matter change.